By Benjamin Britton, Chief Strategy Officer, Ionomr Innovations
For the momentum in green hydrogen to continue, electrolyzer systems must meet targets for capital cost, scalability, and integration with intermittent renewable energy sources, wind and solar. Neither of the incumbent solutions, alkaline (AWE) or proton-exchange membrane (PEMWE) water electrolysis, can claim a confident trajectory to all three. However, alkaline anion-exchange membrane water electrolysis (AEMWE) has emerged as the solution, combining the technologies and the benefits of both incumbent electrolyzer systems for high-efficiency, fully scalable, low-capital-cost electrolyzers.
High current, high efficiency
Green hydrogen production systems are often described by power usage, megawatts or gigawatts, but the more important indicator is what quantity of hydrogen a system can produce (e.g., tons per day), requiring a certain amount of current to pass through the system. The total power draw ultimately depends on the efficiency of this production process. Efficiency in electrolysis is reported as a range because a system can be made to draw variable amounts of current. Hydrogen can be produced in useful quantities between 1.5 V and a typical maximum of 2.2 V, 82% and 53% efficiency (LHV), respectively. More current yields more hydrogen but causes a decrease in system efficiency. The overall cost of hydrogen depends on the efficiency, the ability to vary electricity use, and the capital cost of the system. AEMWE benefits all three.
Today, the main cost of green hydrogen is the electricity, but electricity prices can vary significantly and frequently there is wasted energy when renewable production is high, demand is low, or when production systems are above their grid allotment. This is called ‘curtailed’ or ‘attenuated’ power and with intermittent renewables occurs daily for most of the year. There is a substantial cost incentive to be able to produce more hydrogen when electricity cost is low, and the capability of rapid response is a service to the electricity grid. Both PEMWE and now AEMWE excel in this application.
Capital cost & scalability
The incumbent systems face significant challenges meeting the cost targets and scalability needed for green hydrogen to achieve its potential breadth and impact in the clean energy economy. The clearest path forward was to improve AWE systems, made of earth-abundant metals, to PEMWE efficiencies. AEM electrolysis emerged in the late 2000s as an attempt to realize this vision, with the hope that ion-exchange polymer membranes could one day be made to transport caustic hydroxide ions without the caustic rapidly degrading the functional polymer. Several breakthroughs and over a decade of development later, the first truly stable ion-exchange materials in alkaline-electrolysis conditions are now being produced in large quantities by Ionomr.
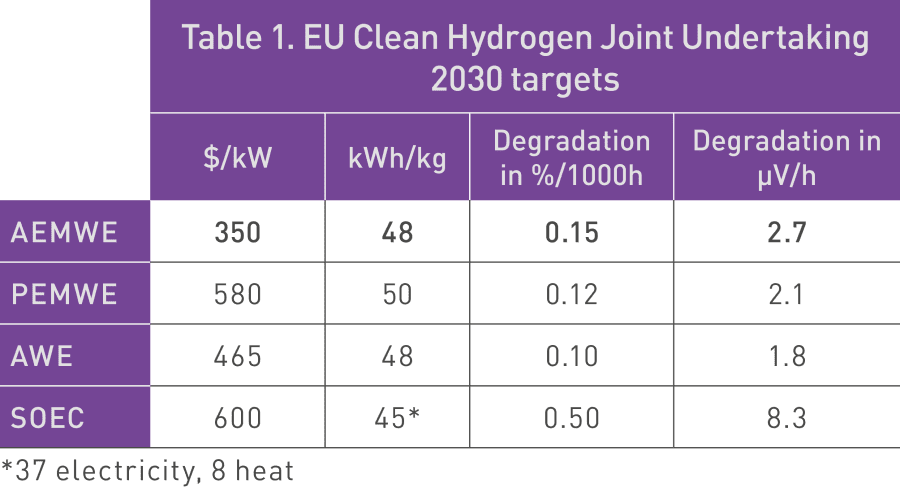
There is now broad recognition of the potential of AEMWE to become the first electrolyzer technology to cross a widely recognized cost target of $350 million per GW, a threshold for true mass production. The Clean Hydrogen Joint Undertaking has recently revealed cost targets for all these technologies (see Table 1). As a result, Ionomr believes AEMWE will enable hydrogen to occupy its role as the clean energy vector of the future.
Alkaline electrolysis (AWE)
Traditional alkaline water electrolysis has been used for industrial hydrogen production since the 1920s. AWE systems operate at low current densities. As a result, these are extremely large systems put together on a project basis in an industrial design and build process. AWE electrolyzers are giant steel ‘stacks’ of hundreds of cells, housed in large warehouses. Individual stacks are sized to the limits of what can be transported, e.g., 40 tonnes and 1.5 meters in diameter by train. Currently, even larger systems are built in ports and transported by ship around the world.
More than any other component, the thick, porous separators, called diaphragms, used in these systems cause high internal resistance and are the origin of the low current densities of AWE, and this is the main aspect AEMWE addresses. Catalysts are the second source of efficiency loss, and improvement is a work in progress – stable catalysts are the origin of the extreme longevity of AWE systems, and the search is underway for materials that do not exhibit the typical trade-off of increased performance and reduced stability.
Single-cell polarization data captures the effects of membrane and catalyst, so it is typically used to compare system efficiencies. However, with AWE there are several additional inefficiencies, seen only at a system level. AWE produces low-pressure hydrogen, and extra compression requires additional energy and capital. The concentrated alkaline electrolytes (e.g., 30–45 wt% / ~6–12 M KOH) causes constant internal electrical shorts called shunt currents. Increasing currents increase the parasitic losses of electrolyte pumping to take heat out of the very large systems. Particularly at low currents, the high gas crossover of the diaphragm results in hydrogen that is ‘recombined’ with oxygen, a further parasitic loss, and the origin of high minimum currents that add to the cost of hydrogen on an annualized basis. More importantly, these require careful pressure balancing to ramp current up and down, necessitating careful control systems for safety and batteries in grid-balancing applications to mitigate the sluggish response.
Recent attempts at improving alkaline electrolysis have included moving to higher pressures and thinner separators to improve efficiency. However, both of these adjustments result in a reduction in safety, especially with the requirement for variable current. Neither are proven in long-duration system and so this avenue remains a significant question. Another improvement is the integration of precious metals in the catalyst meshes, but the still-large areas make the capital effectiveness marginal.

PEM electrolysis
Proton-exchange membrane electrolysis was developed more recently and has become the other established technology by addressing the negatives of AWE, but at an increased cost.
PEMWE employs a cohesive ion-exchange polymer separator that acts as a highly efficient gas and electron barrier, while offering low ionic resistance for up to an order-of-magnitude increase in current density. As a result, stacks are small for the same power, can be containerized for centralized production and easy transportation, and can operate at higher pressures to increase the total efficiency and reduce balance-of-system costs.
However, while the electrolyte is deionized water, the superacid polymer and acidic water-splitting reaction results in a highly corrosive environment. With no electrical connectivity through the electrolyte, catalysis can only occur near the membrane, requiring extremely efficient catalysts. To date, only a platinum cathode for hydrogen emission and iridium anode for oxygen emission offer the requisite performance and durability for industrial systems – an issue for the scalability, cost, and cost risk of these systems. Only ~10 tons of iridium are mined globally per annum, and even ignoring cost risk and with a generous reduction in use per system this provides a hard limit below the growth targets for 2030 and beyond.
An underappreciated aspect of the cost of these systems is the need to coat the titanium structural components in platinum, or sometimes iridium, to achieve durability requirements. As a result, the costs of cell area are very high, and efficiency is sacrificed to get a high current per unit area. This design and ability to achieve dynamic load provides a perfect and safe pairing to intermittent renewables. However, justifying the high capital cost requires a guarantee of long system lifetimes, and this is completely at odds with the need to reduce precious metal content in these systems, creating a dilemma that has no clear trajectory to success.
Alternative green hydrogen

Alternative methods for green hydrogen production include solid-oxide water electrolysis (SOEC) and photoelectrochemical (‘artificial leaf’) systems. SOEC is further ahead of the two but remains expensive (see table 1). This technology shares the challenges of the technology that preceded it, solid-oxide fuel cells (SOFCs). High-temperature gasketing is difficult, operating the systems dynamically causes failures, and systems are difficult to manufacture with the consistency necessary to guarantee long lifetimes.
Photoelectrochemical systems use sunlight and catalysts immersed in water to create hydrogen. These systems are at the early stage of development, have a very large footprint, and would need to operate at near-theoretical maximums to exceed the efficiency of polycrystalline silicon solar + electrolyzer pairings. The only systems with any chance at commercial relevance cogenerate hydrogen and oxygen in the perfect explosive ratio, dangerous to compress and separate. As a result, this technology is unlikely to expand beyond niche applications.
Alkaline AEM electrolysis
Alkaline anion-exchange water electrolysis (AEMWE) is the next-generation electrolysis technology with the potential to disrupt the cost structure of electrolyzers. AEMWE provides a hybrid of the benefits of both incumbent electrolyzer technologies. The design of an AEMWE cell may be conceived either as a significant enhancement to the properties of AWE separators, both in terms of resistance and impermeability, or as a morphing of PEMWE technology that allows a similar form factor and capabilities. A point-by-point comparison is detailed in Table 2.

Compared to PEMWEs, AEMWEs operate in dilute liquid alkaline electrolyte instead of pure deionized water, providing tolerance to impurities, flexible electrode design with a much larger possible active region, and an advantage in large systems. Due to the alkaline environment, the low-cost, non-precious catalysts and components of AWE systems can be employed.
Compared to AWEs, the cohesive, low-resistance membrane of AEMWEs allow efficient operation at high current densities and high pressures, including differential pressures, requiring only one side to have pressure controls. By using dilute electrolyte concentrations (e.g., 1–2 M KOH), parasitic shunt currents are substantially reduced for added efficiency. Overall, the balance of plant (BoP) can be simplified, and safety is increased.

Ionomr’s Aemion+® is the realization of this vision, high performing and completely hydroxide stable in relevant electrolysis conditions, e.g., pH 14 (1 M KOH) and 70–90°C. In ex situ testing, Ionomr’s materials are indefinitely stable. Ionomr’s AEMWE durability demonstration in ‘worst-case’ conditions has exceeded 5,000 hours; by 2,500 hours, the whole-system degradation dropped below 5 µV/h, less than 0.3% loss per 1,000 hours, exceeding 2024 durability targets by more than 3x. Despite the difficult conditions, no losses were attributable to membrane degradation. This is the first demonstration that AEMWE can achieve the same or greater durability than incumbent solutions and that the technology is finally ready to be deployed at the industrial scale.
AEMWE represents a step change in the economic potential of green hydrogen, and the newly developed Aemion+® membranes and ionomers play an enabling role for this revolutionary technology. Ionomr is investing together with pioneering industrial partners globally for the development, scaling, and implementation of AEMWE systems.


