“The biggest hurdle with using seawater is the chlorine, which can be produced as a by-product,” said lead researcher Dr. Nasir Mahmood, a Vice-Chancellor’s Senior Research Fellow at RMIT. “If we were to meet the world’s hydrogen needs without solving this issue first, we’d produce 240 million tons per year of chlorine each year – which is three to four times what the world needs in chlorine. There’s no point replacing hydrogen made by fossil fuels with hydrogen production that could be damaging our environment in a different way.
“Our process not only omits carbon dioxide, but also has no chlorine production.”
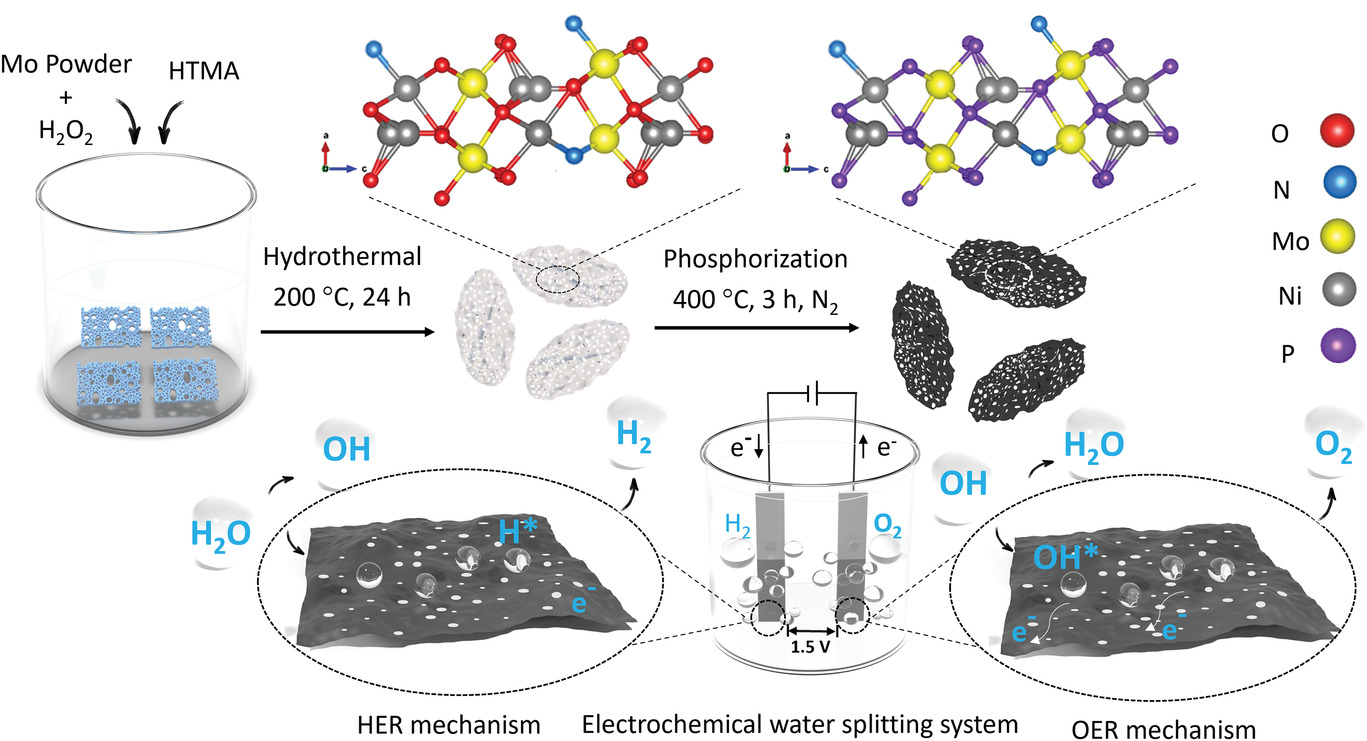
The new approach devised by a team in the multidisciplinary Materials for Clean Energy and Environment (MC2E) research group at RMIT uses a special type of catalyst developed to work specifically with seawater.
The study, with PhD candidate Suraj Loomba, focused on producing highly efficient, stable catalysts that can be manufactured cost-effectively, take very little energy to run and could be used at room temperature.
“While other experimental catalysts have been developed for seawater splitting, they are complex and hard to scale,” said Loomba. “Our approach focused on changing the internal chemistry of the catalysts through a simple method, which makes them relatively easy to produce at large-scale so they can be readily synthesised at industrial scales.”
According to Dr. Mahmood, the technology could significantly bring down the cost of electrolysers – enough to meet the Australian Government’s goal for green hydrogen production of $2/kilogram.
The researchers at RMIT are working with industry partners to develop aspects of this technology.
A provisional patent application has been filed for the new method, detailed in a lab-scale study published in the peer-reviewed Wiley journal Small.
The next stage in the research is the development of a prototype electrolyser that combines a series of catalysts to produce large quantities of hydrogen.