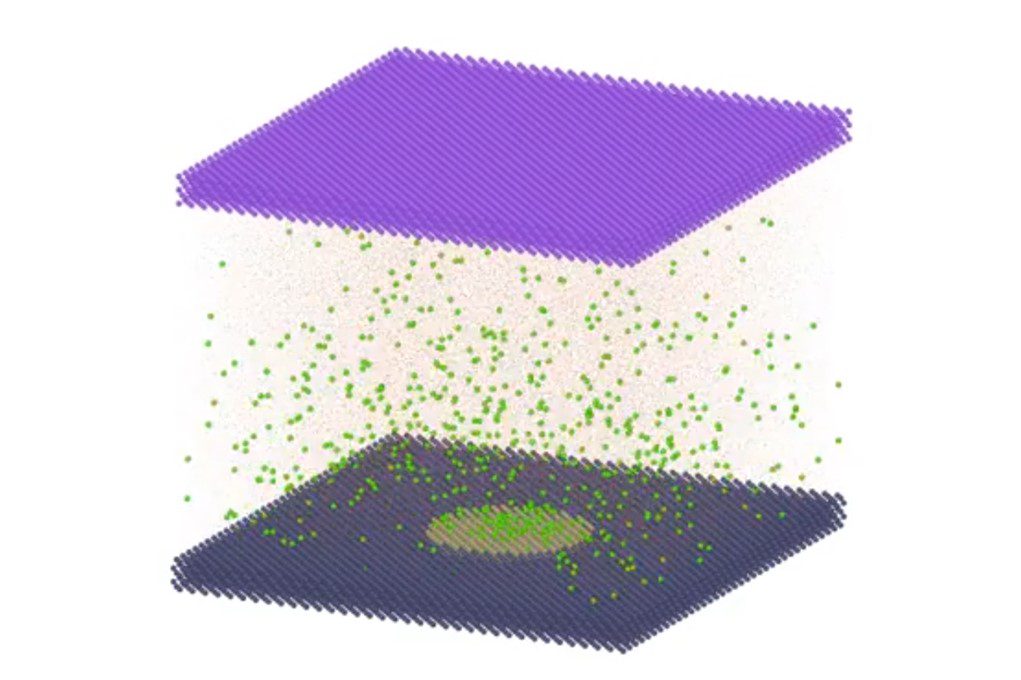
Supported by advanced molecular simulations, Detlef Lohse and his team have developed a theory that can successfully predict the electrical current density needed to allow the nanobubbles to grow uncontrollably and detach, thus freeing the electrode for further hydrogen production. This finding is pivotal as it enables the prediction and control of bubble behaviour, ensuring that electrolysis can proceed with minimal disruption. The research builds upon the established stability theory for surface nanobubbles (the Lohse-Zhang model) and extends it to include the electrolytic current density to predict bubble behaviour.
With this improved knowledge, scientists and engineers can now work towards enhancing the detachment of bubbles. Besides improving the overall efficiency of water electrolysis, this work can also be used in other systems where gas bubbles are formed, such as in catalysis.
The study was published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS).