By Carlos Bernuy-Lopez, Senior Consultant – Power-to-X and Hydrogen Technologies at Ramboll
Introduction
Green hydrogen is poised to play a crucial role in addressing many of the climate challenges we face in the coming years, including the need to reduce greenhouse gas emissions and achieve our climate targets. For instance, if we only consider the current use of hydrogen in the European Union (EU), which is mostly grey hydrogen derived from the steam reforming of natural gas, over 220 metric tons (Mt) of carbon dioxide equivalent (CO2eq) could be avoided, which accounts for 8% of the EU’s total emissions. In addition, green hydrogen is key to decarbonising hard-to-abate industries such as steel manufacturing, which is responsible for emitting over 200 Mt of CO2eq, or 7% of total EU emissions.1,2 Therefore, the EU has set an ambitious target through RePowerEU to produce around 20 Mt of renewable hydrogen by 2030, which could help eliminate a significant portion of these emissions associated with grey hydrogen use and steel production.
The cleanest manner to produce green hydrogen is through electrolysis, which splits water molecules into hydrogen and oxygen in a device called electrolyser. If the electricity powering the electrolyser comes from renewable sources, the process is fully green. Therefore, the more available and affordable renewable energy makes green hydrogen a very competitive solution to both replace grey hydrogen and manufacture green steel. However, the cost of both electricity and electrolysers determines the cost competitiveness of green hydrogen projects, impacting the so-called levelized cost of hydrogen (LCOH). The LCOH takes into consideration both capital and operating expenditures (CAPEX and OPEX) of a certain green hydrogen project and is expressed in €/kg or $/kg.
As key equipment in green hydrogen projects, electrolysers have a major impact on the LCOH, along with the electricity price. Different electrolysis technologies can be used for water electrolysis, each with different characteristics in terms of CAPEX and OPEX. These are alkaline water electrolysis (AWE), proton exchange membrane (PEM) water electrolysis, solid oxide electrolysis cells (SOEC), and anion exchange membrane (AEM) water electrolysis. For all of them, CAPEX is heavily influenced by the materials used, while the electrical efficiency of each technology determines the OPEX associated with carrying out the electrochemical reaction. This article will review the main characteristics of these technologies as well as the key materials forming part of the different electrolyser types. Finally, the article will present LCOH values for state-of-the-art examples of each technology in 2023, as well as expected LCOH values in 2030, based on future projections of electricity prices and improved CAPEX and OPEX for each technology.
Alkaline water electrolysis
Alkaline water electrolysis (AWE) is the most mature electrolysis technology, which uses a liquid electrolyte (KOH). The main characteristics of AWE, along with the current LCOH values that can be obtained with two commonly used reference electricity prices (€60 and €40/MWh), are presented in the first column of Table 1. Both large stacks and systems can be achieved with the use of pressurized alkaline technology: stacks as large as 5 MW with an output pressure of 100 kg/h and systems close to 1 GW. In terms of CAPEX and OPEX, an average CAPEX for an AWE system is around €500/kW or €25,000 kg/h, and the average OPEX is around 54 kWh/kg, with a stack lifetime of 80,000 h.3,4
Regarding the materials used in AWE, both pure Ni and Ni-plated carbon steel are the more common materials, with the use of some expensive and rare-earth metals such as Ru or Ir being significant in some of the solutions offered in the market. Recent calculations made by the International Energy Agency estimate that AWE uses around 800 kg/MW of Ni.5 Pure Ni and Ni-plated carbon steel are used as components of different parts in the electrolyser stack, such as bipolar plates and electrode supports, or even as catalysts in the case of Ni. Ni-plated carbon steel is intended to replace pure Ni in all components where less harsh conditions allow that, and as the quality of the Ni coating over carbon steels is improved. Balance-of-system components, such as electrolyte tanks or gas separators, are mainly made of Ni-plated carbon steel, but due to the corrosion characteristics of the electrolyte, some stainless-steel components may also be needed. In addition, stainless steel is also used for system tubing. Finally, non-expensive catalysts such as Raney Ni, but also Ni, Fe and/or Cu-containing alloys, are the more common materials used as catalysts. In some cases, the use of Ru and Ir can also be found, allowing the operation of the stack at higher current densities, leading to smaller footprints, although without much improvement in electrical efficiencies.
As can be seen from Table 2, with the expected projections for 2030 and lower renewable electricity prices (€15 and €30/MWh as reference), the LCOH can already be quite interesting, and the improvement is much more significant when OPEX (both electricity consumption and durability) is improved. Therefore, it seems an interesting approach to focus less on reducing costs when a good CAPEX level is reached (€300–€400/kW) and instead to improve both electricity consumption and degradation of the AWE stacks. High-quality Ni coatings on carbon steel components leading to lower Ni content will result in good CAPEX levels. Likewise, longer component durability will lead to lower OPEX and, therefore, lower LCOH. Finally, achieving higher electrical efficiency and cost-efficient catalysts with larger surface area will bring electricity consumption down, which will contribute to improving the LCOH (lower OPEX), even without such favourable electricity prices.
PEM water electrolysis
Proton exchange membrane water electrolysis (PEMWE) is characterised by having a solid electrolyte and by operating at much higher current densities, resulting in a significantly smaller system footprint. With a relatively high output pressure of ca. 30 bar, it produces high-purity hydrogen (99.999%). The second column of Table 1 summarizes the main characteristics of this technology, as well as the LCOH calculations for electricity at both €60 and €40/MWh. Rather large stacks can also be achieved, with current sizes averaging 1 MW and 17 kg/h of produced hydrogen. Lower footprints of 25 kg/h per m3, compared to 7 kg/h per m3 in the case of AWE, can be achieved. These large stacks in hydrogen output and small footprints allow PEM manufacturers to currently reach system sizes in the GW range. In terms of CAPEX, PEMWE is about 50% more expensive on average than AWE. This value is €750/kW or €44,000 kg/h, and has slightly higher electricity consumption on average than AWE (56 kWh/kg) as well as a shorter stack lifetime on average (60,000 h).3,4
Regarding the materials, PEM is the more demanding technology in terms of raw materials, as it uses large quantities of Ti, Pt, and Ir. Ti is used in some of the stack components, such as bipolar plates and porous transport layers (PTLs), due to its good performance and stability in the service conditions (high potentials in acidic media). Pt and Ir are used as catalysts to carry out the high-demanding electrocatalytic reaction in acidic media, with loads of Ir and Pt about 0.3 kg/MW and 0.7 kg/MW respectively.5 In addition, Pt is also used as a coating for some of the Ti components described above (mainly PTLs). One of the main advantages of PEM technology is the use of fewer balance-of-system components, as no electrolyte tanks or gas separators are needed. However, the use of stainless steel for system tubing is still necessary, as is the case with AWE technologies.
As in the case of AWE, Table 2 shows the projections for CAPEX, OPEX and the LCOH of PEMWE for 2030. It is expected that there will be a considerable decrease in CAPEX as coated stainless steel components will be able to replace Ti components in both bipolar plates and PTLs. In addition, a decrease in catalyst loading will be achieved for both Pt and Ir due to improved manufacturing techniques that will lead to catalysts with a much larger surface area. This will also improve both electrical efficiencies and durability, decreasing the OPEX. The expected projections for CAPEX and OPEX (€500/kW, 50 kWh/kg and €18,000 per kg/h) will also allow this technology to produce hydrogen with a competitive LCOH.
Solid oxide electrolysis
Solid oxide electrolysers (SOECs) are characterised by their ability to operate at high temperatures (i.e., 500–850°C), making them the most efficient technology of all. Additionally, they are made of cheap and abundant materials (i.e., ceramic oxides). The third column of Table 1 shows the main characteristics of this technology, together with LCOH calculations for electricity at both €60 and €40/MWh. Compared to PEM and AEM, SOECs use much smaller stacks due to the difficulty in scaling up high-quality and reliable ceramic technology. However, these systems can already achieve the MW scale, allowing for their deployment and further development. The main advantage of SOECs over other electrolysis technologies is their much higher efficiency. They operate at the thermoneutral point (1.23 V), resulting in stack efficiency very close to 100%. An average electricity consumption value for SOECs when feeding steam water at 150°C is 40 kWh/kg and 45 kWh/kg when heating of water is considered.3,4 In terms of current LCOH values, this technology offers the best values due to much lower OPEX, even with its shorter lifetime, as its stack replacement is much cheaper than for AEM or PEM technologies.
SOECs are made of cheap and abundant materials, namely ceramic oxides containing inexpensive and readily available materials such as Zr, Fe, Mn, stainless steels. There are also other materials, such as Ce or Y, that are less abundant but still cost-effective and readily available. Special mention must be made of both Ni and Co as both materials are used quite extensively, which could be an issue. However, the current use of Ni and Co is only 200 and 25 kg/MW, respectively, which is four times less than in alkaline technologies in the case of Ni.5 The use of high temperatures is another material concern as more advanced stainless steels need to be used since the operating temperature is higher (i.e., >750°C) in both stack components and hot boxes. However, recent developments show a trend in decreasing the operating temperature below this critical level (<700°C), where cheaper stainless steels can be used.
The table highlights the immense potential of this technology as it can enable us to achieve the cost level of €1/kg of hydrogen by 2030, even with relatively high electricity prices. This is due to the possibility of attaining electrical efficiencies close to 95% if excess heat is supplied to the system. The expected decrease in CAPEX and OPEX (€400/kW, €15,000 per kg/h, and 38 kWh/kg), along with increased durability and larger systems, will help to achieve LCOHs close to €1/kg.
AEM water electrolysis
Anion exchange membrane (AEM) water electrolysis is the least developed of the electrolysis technologies discussed in this article, although it has large potential to contribute significantly to the overall picture. It is a membrane technology, like PEM, but operates in an alkaline medium, allowing for the use of inexpensive materials and resulting in a smaller footprint. The main characteristics of this technology, including its efficiency and electricity consumption, are presented in the last column of Table 1. The table, however, does not show the CAPEX and OPEX values for AEM electrolysers, as there are currently very few companies in the market selling AEM electrolyser products, and the author of this article wishes to present only significant average values for all electrolysis technologies.
Regarding materials, the main advantage of AEM is the use of abundant and cost-effective materials in most of the components. Only excessive use of Ni as the main component could jeopardise the competitiveness of this technology. A current limitation of AEM technology is the lack of cost-effective and long-lasting membranes suitable for use in an alkaline medium. However, rapid developments are being made in this area, which is expected to make AEM a viable solution in the medium term. This is reflected in the promising projections for AEM technology in terms of LCOH, as shown in Table 2.3
LCOH considerations and value chain
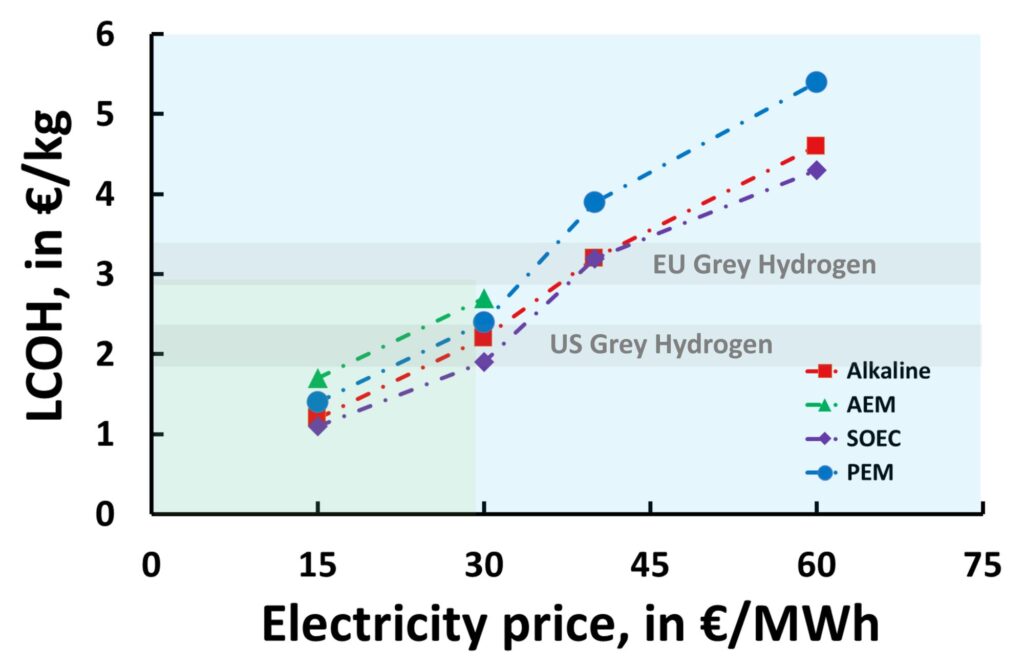
As mentioned at the beginning of this article, electrolysis technologies are an essential component in determining the final LCOH for a green hydrogen project. Figure 1 summarises the LCOHs achievable by using different electrolysis technologies, based on a set of reference electricity prices, both currently and projected for 2030. Encouragingly, all four technologies discussed can reach the LCOH target of less than €2/kg by 2030, which would make green hydrogen competitive with the lowest-cost grey hydrogen currently available in both Europe and the US. This is crucial to achieving the ambitious hydrogen production goals by 2030.
For example, to produce 10 Mt of green hydrogen in the EU, 650–750 GW of electrolysis capacity would need to be installed, depending on the technology employed. A higher penetration of SOECs would potentially reduce this capacity requirement. However, manufacturing several hundred GWs of electrolysers within the next seven years is an optimistic target, given that the current manufacturing capacity worldwide is estimated at only 20 GW. Therefore, the success of achieving our climate goals will depend on the contribution of every manufacturer across all technologies. Fortunately, this represents a great opportunity for developing new industries, economic growth, and job creation as we transition away from fossil fuels.
Conclusion
Four electrolysis technologies can currently be found in the market: alkaline, PEM, SOEC, and AEM. In this article, the current state of the art and challenges, as well as the technological and price developments expected to occur by 2030, have been presented. Based on this analysis, significant growth in deployment of these technologies can be expected in the coming years.
Alkaline technologies are likely to be used in projects that have fewer electricity price restrictions, little space limitations, and low renewable variabilities, such as large, GW-scale industrial projects connected to hydropower. On the other hand, PEM technologies are better suited for projects with high renewable variability and limited space, such as offshore projects. SOEC are ideal for projects with excess heat and lower demand for hydrogen, such as projects in the steel and fertilizer industries, 50 to 100 MW in size.
Even greater deployment of green hydrogen projects can be expected in 2030 and beyond, characterised by lower CAPEX and OPEX, and consequently lower LCOH. This progress will be driven by further reductions in renewable electricity costs and advancements in electrolysis technologies.
About the author