By Katherine Rinaldi, Director of Government Affairs, H2U Technologies
Over 60 million years ago, a giant asteroid collided with our planet and wiped out most life on Earth. Fast forward to today and the material from that extraterrestrial impact still accounts for most of the mineable iridium on the planet. And it also illustrates why the current reliance on iridium in rapidly growing technology markets may become problematic. There’s only a limited supply . . . unless there’s another catastrophe!1
Pivotal role of PEM electrolyzers
Recently, hydrogen has emerged as a promising carbon-free fuel capable of cleaning up difficult-to-decarbonize sectors of the economy, including long-haul transport, aviation, steelmaking, and grid-scale electricity storage. However, one of the key technologies for hydrogen production, electrolyzers, currently relies on iridium. Within a proton exchange membrane (PEM) electrolyzer, iridium acts as a catalyst that drives a chemical reaction and improves overall efficiency. Good catalysts are both highly active and highly stable, producing the maximum amount of product with the minimum energy input and surviving under a normal range of operating conditions for years. To split water into hydrogen and oxygen, electrolyzers need two catalysts, one for each product. The most active and stable catalyst for the oxygen-evolving reaction in a PEM electrolyzer is iridium oxide.
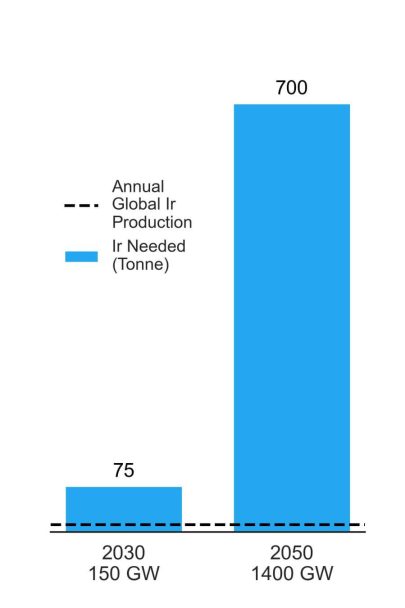
As demand for clean hydrogen increases, so does the need for electrolyzers. In fact, a 2021 report by Morgan Stanley expects global installed electrolyzer capacities to increase to 150 GW by 2030 and 1,400 GW by 2050. With an installed capacity of around 0.3 gigawatts (GW) in 2020, this means they expect a 500x increase in iridium demand for PEM electrolzyers in just the next eight years.2 Of the various electrolyzers available, PEM electrolyzers are particularly suitable for clean energy applications, as they pair well with variable renewable electricity resources like wind and solar. Therefore, much of this installed capacity will be for PEM electrolyzers. However, the scarcity of the catalyst materials used in PEM electrolyzers, particularly iridium, could become a potential bottleneck in the growth of this industry.
State-of-the-art PEM electrolyzers require about half a tonne of iridium per GW of installed capacity.3 Today, the global annual production of iridium is around nine tonnes per year.4 At that production rate, it would take over 77 years of using all the annual supply of iridium, completely disregarding all other industrial uses, to reach the estimated 2050 installed capacities of electrolyzers. In other words, there is simply not enough iridium on Earth to develop PEM electrolyzer technology at the rates necessary to match the expected growth.
Methods to reduce iridium dependence
Thankfully, talented scientists, engineers, and industry leaders are already working on a variety of solutions to tackle this problem. One potential pathway is to reduce the amount of iridium in an electrolyzer. Lowering the catalyst loading without sacrificing performance or durability is a significant challenge, but if researchers can increase the effective surface area of the catalyst material, this goal can be achieved. Techniques to increase surface area include coating the material on a structured, high-surface-area support or synthesizing high-surface-area catalyst particles directly. Results from this field of research have shown promise in reducing catalyst loadings up to a factor of ten in laboratory settings, but further research is needed to evaluate their longevity and performance in industrial settings and under real operating conditions.5
Another potential solution is to recycle the iridium at the end of an electrolyzer’s life. Because the catalyst is not consumed during electrolysis, the precious material can be partially recovered when an electrolyzer is retired and recycled for future use. Although current recycling rates of iridium are low, some researchers predict that the end-of-life recycling rate of iridium could increase to around 90%, similar to other precious metals like platinum and palladium. This could help address the iridium problem in the long term, when there is more electrolyzer capacity to recover material from after use.6
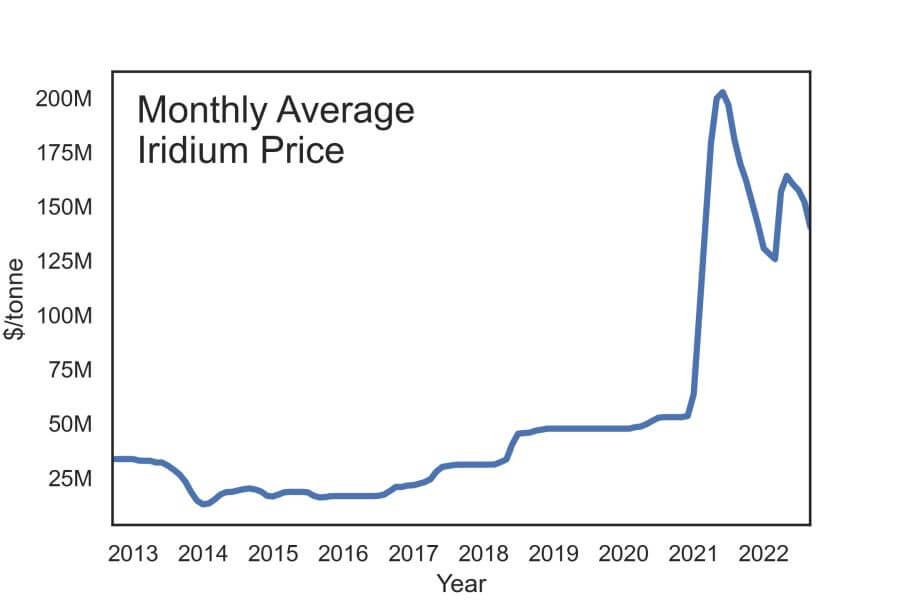
Both of these solutions can reduce strain on global iridium supplies during the growth of the electrolyzer market. However, while iridium’s scarcity imposes harsh limitations on future electrolyzer development, its price volatility could be a larger risk in the near term. With a limited global supply and multiple novel technologies that rely on the material, including electrolyzers and 5G infrastructure, iridium is subject to major fluctuations in price over time. Efforts like recycling of or reducing the amount of iridium can lessen the impact of price volatility on overall electrolyzer costs but cannot eliminate the problem entirely. Solutions that offer alternatives to iridium, however, could both minimize the issue of cost in the near term and relieve pressure on global iridium supplies in the long term.
New catalyst discovery
Novel catalyst development targets three often competing objectives: to reduce material costs, to increase stability, and to increase activity. For the PEM electrolyzer industry, the importance of each of these objectives can vary with the use case. For example, for energy storage applications, capital costs contribute more substantially than electricity costs to the overall cost of the electrolyzer. In this use case, cheaper but less efficient catalysts could provide more economic benefit than more expensive, more efficient ones.7 Discovering a diverse family of catalysts to replace iridium oxide could be the essential key to enabling a hydrogen economy.
As a case in point, research from the California Institute of Technology (Caltech), backed by a USD 122 million Joint Center for Artificial Photosynthesis (JCAP) program established by the Department of Energy and a group of universities, including Caltech, University of California, Berkeley, Stanford University, UC Irvine, and UC San Diego, has led to the discovery of an important tool: a rapid catalyst discovery system. The mission of the JCAP was to find cost-effective methods to produce fuels using only sunlight, water, and CO2. This program produced over 30 patents and has led to the development of a unique, high-throughput catalyst discovery system. This data-driven process focuses on the discovery of new, non-rare earth catalysts for the generation of clean fuels. Using this method, scientists synthesize, characterize, and quantify the catalytic activity of thousands of material compositions per week and then close the loop with big data analysis to refine and guide the search.
A key technology within the catalyst discovery system is the scanning droplet cell (SDC), a custom-built device that performs electrochemical experiments in a single droplet of liquid. To use the SDC, scientists first deposit catalyst samples in an array of about 1 cm2 spots on a conductive substrate. They then load this substrate onto the sample stage and define the area of the SDC experiment by maintaining a liquid droplet on the substrate that matches the size of the catalyst spot. At each spot, they perform small-scale, 3–10 second electrochemistry experiments that reveal the activity of each catalyst. Using motion-controlled stages, they then move the substrate so that the droplet is over a new catalyst material. In this way, they can rapidly analyze an entire plate of hundreds of catalyst materials in a few hours. This technique screens catalyst materials faster than any other on earth, making it a highly valuable tool in the ongoing search for iridium replacements.
Conclusion
The problem of iridium scarcity poses a major risk to the development of a large electrolyzer market. Methods to both reduce the industry’s reliance on this rare earth material and develop lower-cost alternatives must be established over the next few years for the industry to grow to meet rising demand. If we’re lucky, no new iridium will be coming from space to our planet anytime soon, so in the meantime, we’ll have to explore these promising solutions to solve the iridium problem for ourselves.
About the author

Katherine Rinaldi is the Director of Government Affairs at H2U Technologies. Prior to this position, Katherine earned her PhD in Chemistry at the California Institute of Technology, where she studied the role of hydrogen in renewable energy systems from both a technical and systems perspective. She initially joined H2U Technologies as a catalyst engineer, during which time she helped to construct and operate the catalyst discovery engine at its facilities in Chatsworth, California.
* Opening image by D. Nishio-Hamane, www.flickr.com