The new method was developed by doctoral student Itzhak Grinberg and Dr. Oren Ben-Zvi, under the guidance of Prof. Iftach Yacoby of the School of Plant Sciences and Food Security at the Faculty of Life Sciences and Prof. Lihi Adler-Abramovich of the School of Dental Medicine and the Center for Nanoscience and Nanotechnology. The promising research results were published in the journal Carbon Energy, focusing on advanced materials and technology for clean energy and CO2 emission reduction.
“Hydrogen is very rare in the atmosphere, although it is produced by enzymes in microscopic organisms, which receive the energy for this from photosynthesis processes,” explained Itzhak Grinberg. “In the lab, we ‘electrify’ those enzymes, that is, an electrode provides the energy instead of the sun. The result is a particularly efficient process, with no demand for extreme conditions, that can utilize electricity from renewable sources such as solar panels or wind turbine. However, the enzyme ‘runs away’ from the electric charge, so it needs to be held in place through chemical treatment. We found a simple and efficient way to attach the enzyme to the electrode and utilize it.”
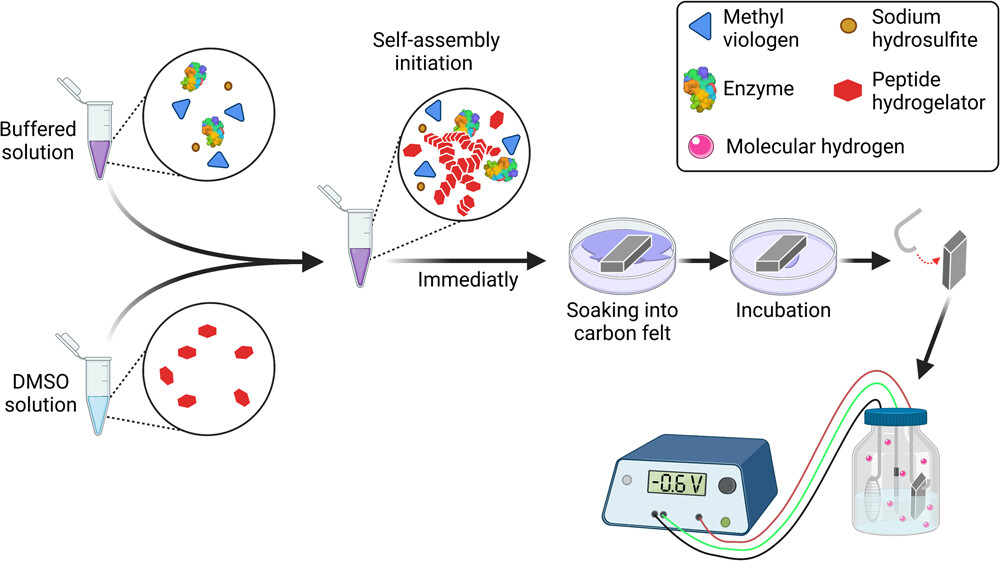
The researchers used a hydrogel (a water-based gel) to attach the enzyme to the electrode and were able to produce green hydrogen using a biocatalyst, and with over 90% efficiency; that is, over 90% of the electrons introduced into the system were deposited in the hydrogen without any secondary processes.
“The material of the gel itself is known, but our innovation is to use it to produce hydrogen,” explained Prof. Iftach Yacoby. “We soaked the electrode in the gel, which contained an enzyme for producing hydrogen, called hydrogenase. The gel holds the enzyme for a long time, even under the electric voltage, and makes it possible to produce hydrogen with great efficiency and at environmental conditions favorable to the enzyme – for example, in salt water, in contrast to electrolysis, which requires distilled water.”
Prof. Lihi Adler-Abramovich added: “Another advantage is that the gel assembles itself – you put the material in water, and it settles into nanometric fibers that form the gel. We demonstrated that these fibers are also able to stick the enzyme to the electrode. We tested the gel with two other enzymes, in addition to the hydrogenase, and proved that it was able to attach different enzymes to the electrode.”
“Today, ‘green’ hydrogen is produced primarily through electrolysis, which requires precious and rare metals such as platinum along with water distillation, which makes the green hydrogen up to 15 times more expensive than the polluting ‘gray’ one,” said Dr. Oren Ben-Zvi. “We hope that in the future, it will be possible to employ our method commercially, to lower the costs, and to make the switch towards using green hydrogen in industry, agriculture, and as a clean energy source.”
Opening image: Schematic diagram of electrode preparation. Credit: Carbon Energy